If Blood Cells Are Placed in a Hypertonic Solution
W hen a red blood cell is placed in a hypertonic solution such as a highly saline environment there is a lower concentration of solute particles inside the cell than outside in the extracellular space. FYI hypotonic solution has lower concentration of solutes and high concentration of water so if a red blood cell is put in hypotonic solution the water would move in and the cell would burst.

State True Or False Rbc On Keeping In Hypertonic Solution Shrinks Down
If enough water is lost the cell will take on a wrinkled or shriveled appearance.
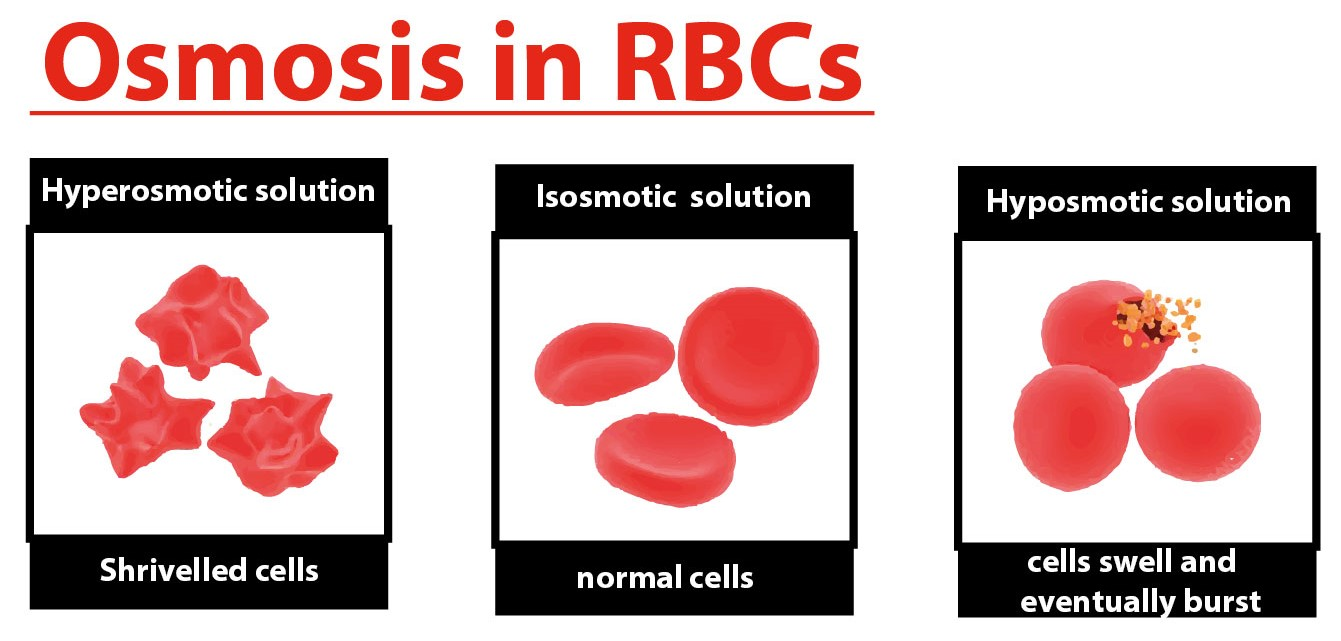
. Isotonic solution the flow of water in and out cell is happening at the same rateOne red blood cells Swells and hemolyses bursts when placed in a container hypotonic solution. In the case of a plant cell however a hypotonic extracellular solution is actually ideal. If blood cells are placed into a hypertonic solution the cell will lose water and shrink as the water moves down its concentration gradient from the cell into the solution.
If blood cells are placed in a hypertonic solution asked Dec 31 2020 in Biology Microbiology by crystalbeauty412 a. If a cell is placed in a hypertonic solution the cell is considered. Animal cells tend to do best in an isotonic environment where the flow of water in and out of the cell is occurring at equal rates.
If the same blood cell is placed in ahypotonic solution the blood cell grows in size. When placed in a hypertonic solution a red blood cell will lose water and undergo crenation shrivel. Feb 15 2018 A cell loses its H 2O molecules when it is placed in hypertonic solution.
If blood cells are placed in a hypertonic solution A. When placing a red blood cell in any hypertonic solution there will be a movement of free water out of the cell and into the solution. Why do red blood cells become Crenated in hypertonic solution.
A hypertonic solution means that there is more salt in the solution or external environment than within the red blood cells. What will happen if red blood cells are placed in a hypertonic solution. Which means that a solution contains less water as compared to the water within the cell.
If the cell was placed in hypertonic solution water would have moved out of the cell causing it to shrink. Cthe cells will swell due to diffusion. A hypertonic solution has a higher solute concentration compared to the intracellular solute concentration.
When placed in a hypertonic solution a red blood cell will lose water and undergo crenation shrivel. B The cells remain unchanged due to equal water concentrations inside and outside the cells. The red blood cells undergo crenation which means they shrink and shrivel as water leaves the cells until the concentration.
If a cell is placed into a. The cells will burst due to active transport. When placed in a Hypertonic solution One red blood cells Moisture will be.
The cell membrane of RBC acts as a selectively permeable. In the case when the cell should be placed in the hypertonic solution so it will be shrink because of the water loss from the cell. Therefore a hypertonic solution has more solutes than the intracellular environment so water will leave the cell to try to achieve equilibrium.
As water leaves the cell it shrinks and develops the notched appearance characteristic of crenation. Click to see full answer. A hypertonic solution contains a higher concentration of solutes compared to another solution.
If placed in a hypotonic solution a red blood cell will bloat up and may explode while in a hypertonic solution it will shrivelmaking the cytoplasm dense and its contents concentratedand may die. A solution with a lower concentration of water and a higher concentration of solute in comparison to another solution it is called a hypertonic solution. The cells will shrink due to water loss by the cell.
If a cell is placed in a hypertonic solution water will leave the cell and the cell will shrink. To determine the effect of osmotic pressure on red blood cells RBCs of own blood sample. Red blood cells do not have the cell wall and contractile.
What is hypertonic solution. A blood cell placed in hypotonic solution would gain water as water will enter cell from surrounding hypotonic medium by the process of osmosis causing the cell to swell up. Hypertonic Solution Definition.
The hyperonic solution should represent the high solutes content that. Bthe cells will be unaffected since they have a cell membrane to separate them from the solution. If there is a higher concentration of solutes outside of the cell than inside it such as would happen if you placed red blood cells in a concentrated salt solution then the salt solution is hypertonic with respect to the inside of the cells.
In red blood cells this is called crenation and the surface of the cells take on a scalloped appearance. Hypertonic solution is the one which contain more concentration of solutes as compared to the concentration of solutes in cytoplasm of cell. Red blood cells placed in a solution with a higher water concentration compared to their contents eg pure water will gain water by osmosis swell up and burst.
When a red blood cell is placed in a hypertonic solution it shrinks as water is drawn out of the cell and into the surrounding solution. When red blood cells are placed in a hypertonic solution water within the cells move out via osmosis into the surrounding solution causing the red blood cells to shrink and shrivel. Disposable needle 26G dropper microscope 70 alcohol or any other suitable marketed antiseptic glass slide and coverslip isotonic 09 NaCl hypertonic hypotonic solution.
Dthe cells will shrink due to water loss by the cell. If placed in a hypotonic solution a red blood cell will bloat up and may explode while in a hypertonic solution it will shrivelmaking the cytoplasm dense and its contents concentratedand may die. In the case when the blood cell should be placed in the hypertonic solution so the cells should be shrink because of the water loss via the cell.
If a cell is placed into a hypotonic solution the water will flow into the cell causing it to swell and possibly lyse. If red blood cells are taken from the body and placed in a hypertonic solution what happens to the cells. A The cells remain unchanged due to equal solute concentrations inside and outside the cells.
A high amount of water loss can be. If a red blood cell is put in hypertonic solution the water from red blood cell moves out to equalise the water concentration in hypertonic solution and thus the red blood cell shrivel. C They become white blood cells.
Summary of Red Blood Cell Placed into Hypertonic Isotonic and Hypotonic Solutions. The opposite solution with a lower concentration is known as the hypotonic solutionScientists must describe cell contents compared to the environment. What happens when we place blood cells in water give reason.

What Will Happen When Red Blood Cells Are Placed In Class 12 Biology Cbse
What Would Happen To An Rbc Placed In A Hypertonic Solution What About A Hypotonic Solution Quora
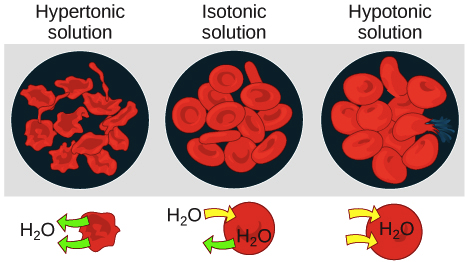
Tonicity Hypertonic Isotonic Hypotonic Solutions Article Khan Academy
Comments
Post a Comment